With the continuous advancement of technology, solventless lamination is welcomed by most of flexible package manufacturer.
Faster, easier, more environmentally friendly, more cost-effective are the advantages of solventless lamination.
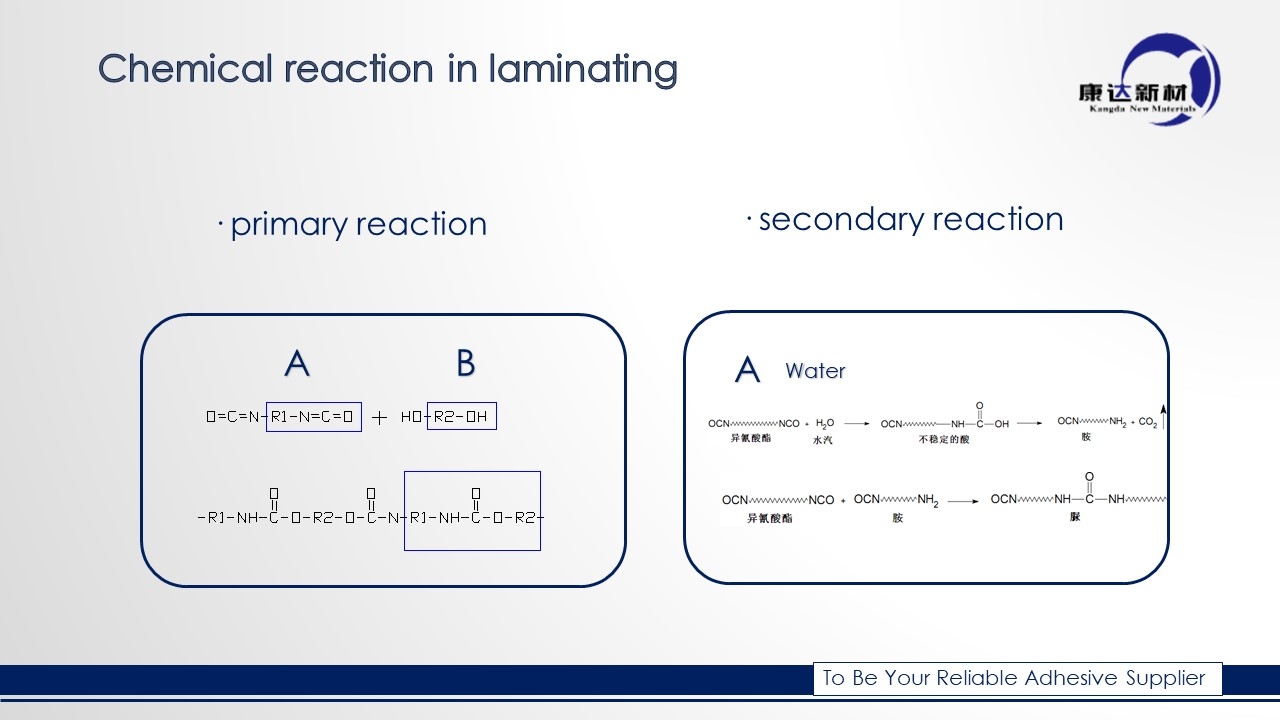
It’s very important for us to know the basic chemical reaction during solventless lamination for better mass production.
Two component Solventless adhesive was made by polyurethane (PU), PU was combined by isocyanate(-NCO) most called A component, and polyol(-OH) mostly called B component. The details of reaction please check in below;
The primary reaction is between A and B, the -NCO have chemical react with -OH, at same time, due to water also have the -OH functional group, water will have chemical reaction with A component release the CO2,Carbon dioxide. And polyurea.
The CO2 may cause the bubble problem and polyurea may cause the anti-heat seal. Besides if the humidity high enough, the water will consume too many A component. The result is that adhesive can’t cured 100% and the bonding strength will lower.
In summary, we suggest that;
The storage of adhesive should be Stored in a cool, dry place away from moisture
The workshop should keep humidity between 30%~70%, and use AC to control the humidity value.
Above are the basic chemical reaction between two component adhesives, but mono-component adhesive will totally different, we will introduce mono component chemical reaction in future.
Post time: Dec-07-2022